Safety and Efficacy Are the Core of Our Practice
One of the most frequent questions I get asked in meeting with corporate clients is:
Are the Regenexx Procedures performed in the United States FDA approved?
The Food and Drug Administration does not “approve” or “not approve” medical procedures performed by physicians. Since Regenexx procedures are a medical procedure and they are performed on the same day, they fall under the same surgery exemption 1271.15(b). Procedures are compliant with CFR 21 Part 1271 which addresses human cells, tissues and cellular and tissue-based products.
The procedures performed in the United States are FDA compliant.
This is important to recognize as more providers are practicing regenerative medicine without the compliance and guidelines in place to ensure patient safety and procedure efficacy. This standard of care, along with a national network of standardized procedures, is a distinct practice that makes us the leader in the industry.
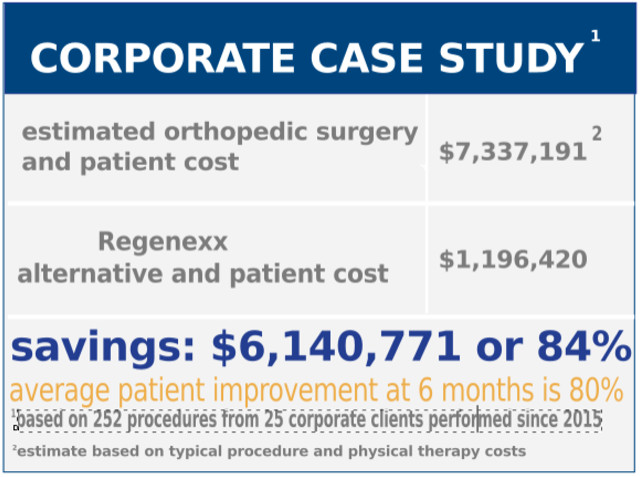
As of February… We have reduced our corporate partners’ orthopedic surgical costs by up to 70%* at an overall savings of over $6.1 MILLION dollars with an average patient improvement at 6 months of 80%.
Watch this video to learn how stem cells became an orthopedic solution and hear Chief Medical Officer, Dr. Chris Centeno, describe the impetus for stem cell treatments in the United States.
Promoting Your Employee’s Well-Being through the Nation’s Leader in Regenerative Medicine
FDA Specific Articles From Regenexx®
Using Common Orthobiologics to Explain the FDA 1271 Regulations
FDA Crackdown on the Stem Cell Wild West
Are Amniotic and Cord “Stem Cell” Products FDA Approved?
The healthcare cost revolution is happening as employers recognize they have to change the trajectory of their healthcare expenses. Incorporating the Regenexx procedures into your healthcare plans is a win for employers and a win for employees. I look forward to having the conversation with you about how we can help.
* This data was calculated based on the gross savings realized from substituting percutaneous orthobiologic procedures for invasive, elective orthopedic surgeries within our pilot program in Des Moines, IA. Now that we have more data from more clients, we will update our cost savings on a quarterly basis using a sampling methodology, later this year we will be introducing an actuarial based process.